THE ROLE OF GENETICS
Genes are made up of DNA. They are the basic units inside a cell by which we inherit traits from our ancestors and pass down traits to our children. Our genetic information, found in our DNA, determines much about us: for example, our eye and hair color. Genes can be associated with diseases, and cancer (of all types) is a disease associated with changes in our genes. A number of genes have been identified that lead to a particularly increased risk of developing cancer in the colon or rectum. While not every hereditary colorectal cancer syndrome has yet to be associated with a specific abnormal gene or gene “mutation,” information about the genes that lead to these syndromes has increased dramatically over the last 20+ years.
This summary is intended for anyone wishing to learn more about hereditary colorectal cancer syndromes and genetic testing for these syndromes. This summary should help the reader understand:
- The definition of a syndrome and specific examples of hereditary colorectal cancer syndromes and their classification;
- How frequently these syndromes occur and how they are diagnosed/identified;
- The symptoms and signs of the diseases that may be associated with these syndromes;
- The benefits of genetic counseling and how best to determine whether genetic testing should be performed.
DEFINITION OF HEREDITARY COLORECTAL CANCER SYNDROMES
Colon and rectal surgery, like many fields in medicine, has seen an incredible increase over the last two decades in the amount of information available about genes and how “problems” with them may lead to cancer. Some of these abnormal genes or gene “mutations” that have been discovered may lead to a “syndrome.” A syndrome is defined as a group of symptoms or signs that occur together and characterize a disease process.
Some of the syndromes that have been discovered can increase an individual’s risk to develop colorectal cancer. These “hereditary colorectal cancer syndromes” are thought to account for up to 10% of all colorectal cancers that are diagnosed, with another 20% having an increased rate of colorectal cancer in their family without a clear hereditary syndrome being found. Not all of these syndromes have yet to be linked to an identifiable abnormal gene or gene mutation. Therefore, the hereditary colorectal cancer syndromes are often diagnosed by the symptoms and signs that they cause, which can be most easily divided or categorized into those with multiple colonic polyps and those without multiple polyps.
SYNDROMES WITH MULTIPLE POLYPS (“POLYPOSIS”)
Familial Adenomatous Polyposis (FAP): While polyposis syndromes are relatively rare, the most common type is “FAP,” characterized by having 100 or more polyps in the colon and rectum.
The polyps found in this condition are a particular type known as “adenomas”, and thus the “adenomatous” term in the name of the condition. Adenomatous polyps have an increased risk of developing colon and/or rectal cancer in general. In FAP, especially with the very high numbers of polyps, this risk can be as high as 100% over a person’s lifetime. Without treatment, the average FAP patient will have colorectal cancer diagnosed by age 40 and up to 7% of the cancers will be diagnosed before age 21. The syndrome does have a 100% chance of developing polyps (if the patient has a gene mutation, they will develop polyps) with the polyps starting to develop on average around the time of puberty (age 16).
The gene mutation that most often leads to this condition is found in a gene that is responsible for a number of important cell functions including suppressing the growth of tumors (called a tumor suppressor gene), named the “APC” gene. If someone has a mutation in this gene, they have a 50% chance of passing the abnormal gene on to their child (this is known as “autosomal dominant”).
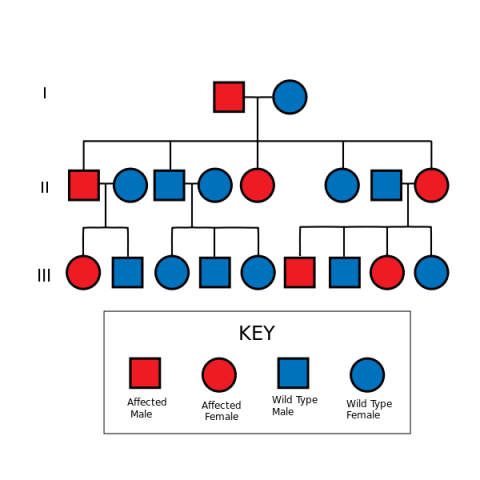
[File: Autosomal Dominant Pedigree Chart.svg]
However, in 20-30% of cases, FAP patients will have a spontaneous gene mutation – meaning that they are the first ones in their family to have the abnormal gene that leads to the condition. The more intense the degree of polyposis in a patient, the more likely an APC gene mutation will be identified, but not all patients with this condition have been found to have a mutation in this gene. For example, 80% of patients with >1,000 polyps are identified with an APC mutation, while less than 60% of those with between 100 and 1,000 polyps will have an APC mutation.
Because the polyps start to form in the colons of patients with FAP at an early age, colonoscopy or sigmoidoscopy screening (using a lighted, flexible scope to examine the lining of the colon and rectum) should begin at age 10-12 in those patients with a known gene mutation or those who are at risk for having a mutation but have not yet undergone gene testing. These scopes should continue yearly until polyps are confirmed, thus confirming the diagnosis of FAP, or until gene testing is performed and shows a negative result in a member of a family with a known APC mutation. A positive scope (i.e., presence of polyps) or genetic test results require continued scoping and/or surgery for the colon and rectal polyps related to FAP.
The APC gene mutation can also lead to a risk of developing other symptoms or signs associated with FAP. Some of these include problems with the teeth, eyes, and bones. Other findings, like “desmoids,” which are fibrous or scar-tissue-like tumors that can occur anywhere in the body, are seen in 15-30% of FAP patients and can lead to significant and life-threatening problems, including bowel blockage or bowel erosion and leakage. Desmoids can require surgery and/or medications when they become too large or cause significant symptoms. Other serious conditions associated with FAP include polyps or cancers in other parts of the intestines and stomach, thyroid cancer, pancreas cancer, and even liver tumors in children. Strategies exist for watching for some of these issues related to FAP, and it is important for FAP patients to consult with their surgeon or primary care giver about these tests.
The primary treatment for FAP is surgery. Scopes, as mentioned above, sometimes offer a chance to remove some, if not all, of the polyps in the colon and rectum and may help to minimize cancer developing in some FAP patients or allow for delaying surgery, but should not be viewed as a means of avoiding surgery altogether. Surgical options are the mainstays of treatment and involve removing all of the colon and connecting the small intestine to the rectum or removing all of the colon and rectum with either a permanent ostomy bag on the abdominal wall (an “ileostomy” — when the last portion of the small intestine is brought out to the skin of the belly to drain into a bag stuck to the skin) or with creation of a small intestine pouch that is connected to the anus (an “ileal pouch anal anastomosis” or “IPAA”, also commonly known as a “J-pouch”). A colon and rectal surgeon can explain the details and risks and benefits of these procedures and help determine which FAP patients may be candidates for each procedure.
Multiple pictures at: http://www.hopkinsmedicine.org/healthlibrary/conditions/adult/digestive_disorders/hereditary_nonpolyposis_colorectal_cancer_treatment_22,HereditaryNonpolyposisColorectalCancerTreatment/
More specific:
http://www.hopkinsmedicine.org/healthlibrary/GetImage.aspx?ImageId=290573
http://www.hopkinsmedicine.org/healthlibrary/GetImage.aspx?ImageId=290579
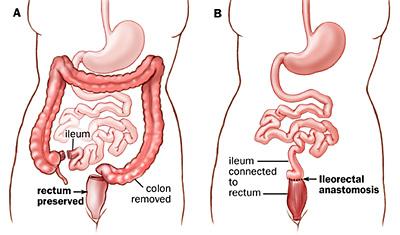
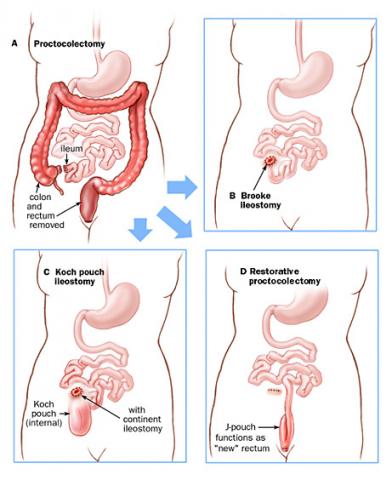 Selecting an appropriate surgical procedure for an FAP patient depends on multiple factors, including the number of rectal polyps that they have; the type of APC mutation that they have; the presence of colon or rectal cancer at the time of the operation; family patterns of the polyps associated with the FAP and family experience with any of the surgery options; the patient’s willingness and ability to undergo future scopes to check any areas of remaining colon or rectum that may develop or have polyps; any concerns about desmoids; any concerns about the patient wanting to have children in the future; and the patient’s health and ability to control their bowels prior to any surgery.
Selecting an appropriate surgical procedure for an FAP patient depends on multiple factors, including the number of rectal polyps that they have; the type of APC mutation that they have; the presence of colon or rectal cancer at the time of the operation; family patterns of the polyps associated with the FAP and family experience with any of the surgery options; the patient’s willingness and ability to undergo future scopes to check any areas of remaining colon or rectum that may develop or have polyps; any concerns about desmoids; any concerns about the patient wanting to have children in the future; and the patient’s health and ability to control their bowels prior to any surgery.
Timing of the operation – how old the patient is when they have the surgery – depends on how extensive the patient’s polyps are; the age of the patient (usually the operations are performed in the late teens or early twenties for those patients diagnosed early in life, assuming they are able to understand the risks and benefits of the surgery); whether and when the patient wants to have children; family patterns of early polyp or cancer development; the patient’s cancer fears; any concerns about desmoids; and the presence of cancer or significant pre-cancerous change in any of the polyps. All of the operations can be performed in a traditional “open” technique (with a longer incision on the abdomen) or laparoscopically (“minimally invasive”), as is determined to be best by the patient and the surgeon.
ATTENUATED FAMILIAL ADENOMATOUS POLYPOSIS
The “attenuated” type of FAP (“aFAP”) has fewer polyps in the colon and rectum (between 10 and 100 polyps with the average patient having between 20-30 polyps). In many cases, this syndrome is due to a mutation in the APC gene, but fewer patients with aFAP are actually identified with a known gene mutation than in FAP. As with FAP, some patients with aFAP are the first in their families to have the abnormal APC gene, while other cases may due to a mutation in a gene called MutYH (see below).
The polyps in aFAP are more often found in the right side of the colon and are less often found in the rectum. Patients with aFAP tend to develop polyps at a later age (35-45 years old) than in FAP and develop their first colorectal cancer at a later age (55-59 years old) when compared to FAP. Because of the polyp locations in aFAP, sigmoidoscopy is not recommended to track the polyps. Instead, colonoscopy is recommended to begin at age 20-30, or ten years sooner than the first polyp has been diagnosed in the family, whichever is first.
While disease-related problems outside of the colon and rectum are not as extensive or frequent, aFAP patients do frequently have upper intestinal polyps (in the stomach and small intestine) requiring surveillance, as with FAP. Because the colonic polyps in aFAP may be manageable with removal during colonoscopy, prophylactic removal of the colon and/or rectum may not be necessary for all patients. Polyp prevention with medications such as those used in FAP may be effective, but larger trials have not been done. The surgical options, indications and considerations are otherwise the same as with FAP. 5,6
MUTYH-ASSOCIATED POLYPOSIS (or “MAP”) is a syndrome caused by a mutation in the MutYH gene that normally helps to repair damage to DNA. In MAP, the patient has to receive an abnormal MutYH gene from both their mother and their father (“autosomal recessive”) for the syndrome to develop. MAP patients usually have a clinical picture very similar to aFAP (between 10 and 100 colon and rectal polyps), but almost 20% of those patients who come to a doctor with more than 100 polyps actually are due to a MAP. The condition usually starts when patients are in their 40s or 50s, and approximately 50% of those diagnosed with MAP will initially present with colorectal cancer.
The colonic polyps found in MAP may be manageable without surgery at least initially, and colonoscopy is recommended to start by age 20-30 (or ten years earlier than the first family diagnosis, whichever is earlier) and continue yearly if needed. While not as common as with FAP and aFAP, small intestine polyps may be present, so at least an initial screening upper scope is also recommended.
SERRATED POLYPOSIS SYNDROME is a hereditary colorectal cancer syndrome associated with polyps known as “sessile serrated polyps,” “sessile serrated adenomas,” and/or “hyperplastic polyps.” Serrated polyps are named based on their knife-blade or jagged appearance under the microscope. This syndrome does not have a well-defined gene mutation associated with it (although it may be associated with mutations in a gene called BRAF that is involved in cell growth signals), so it is defined based on the multiple and/or large serrated-type polyps in the colon and rectum. The colorectal cancer risk with this syndrome is not exactly known, but it is elevated and estimated to be between 7-40%.
Colonoscopy recommendations to track the polyps are on a case-by-case basis depending on the numbers and types of polyps, but it is thought best to start by age 40 (or ten years earlier than the youngest relative in the family diagnosed with the syndrome, whichever is earlier). As with the other polyposis conditions, surgery is recommended for serrated polyposis when the polyps cannot be sufficiently treated with the colonoscope, the polyps cause symptoms (like significant bleeding), or cancer or pre-cancerous changes develop in the polyps. The surgeon will help to determine the appropriate operation.
HAMARTOMATOUS POLYPOSIS SYNDROMES are very rare and have unique polyps called “hamartomas” that can be found in the small intestine, colon or rectum in varying degrees. Included in these syndromes is “Peutz-Jeghers Syndrome” caused by a mutation in the STK11/LKB1 gene (a tumor suppressor gene – which normally prevents cells from dividing in an uncontrolled fashion) with a colorectal cancer risk between 10-39%. “Juvenile polyposis syndrome” is caused by a mutation in the SMAD4 or BMPR1A genes (genes involved in many cell functions), with a colorectal cancer risk between 10-50%. PTEN syndromes (including “Cowden syndrome” and “Bannayan-Riley-Ruvalcaba syndrome”) are hamartoma syndromes associated with a mutation in the PTEN gene (a tumor suppressor gene) and have at least a 9% risk of colorectal cancer.
All of these syndromes are associated with a variety of other cancers and other signs and symptoms. Colonoscopies for the hamartomatous polyposis syndromes should occur every 2-3 years starting in the patient’s teen years. Like many of the other polyposis syndromes, surgery is recommended for hamartomatous polyposis when the polyps cannot be sufficiently treated with the colonoscope, the polyps cause symptoms (like bowel blockage or significant bleeding), or cancer or pre-cancerous changes develop in the polyps. The surgeon will help to determine the appropriate operation.
NONPOLYPOSIS COLORECTAL CANCER SYNDROMES
Originally described by Dr. Henry Lynch in the early 1970s as the “family cancer syndrome,” this is the most common hereditary colorectal cancer syndrome, accounting for 3-6% of all colorectal cancers diagnosed in the United States. It is associated with high risks of colorectal and multiple other cancers. Now known as “hereditary nonpolyposis colorectal cancer” or “HNPCC” (originally known as “Lynch syndrome”), this describes patients with a known gene mutation of four major DNA mismatch repair genes (known as MLH1, MSH2, MSH6, and PMS2) and/or who fit particular clinical criteria (“Amsterdam I and II criteria” or “Bethesda” and “revised-Bethesda criteria”, see Tables 1 and 2). Unfortunately, 40% of those patients who fit the clinical criteria will not have a gene mutation when tested. HNPCC shows an autosomal dominant inheritance pattern (50% chance of passing the abnormal gene on to a child), with a lifetime risk of developing colorectal cancer between 30-72%.
On average, patients with this syndrome are diagnosed with colorectal cancer in their early to mid-40s with some studies showing colorectal cancer risks increasing instead in the early 60s, depending on the gene mutation. The colorectal cancers in HNPCC are usually (60-70%) in the first (i.e., right-sided) portions of the colon and have high rates of multiple colorectal cancers appearing at the same time (5-20%) or developing new cancers in the future (10-50%). There is also a high association with other types of cancers, including those of the uterus (20-60%), stomach (13-19%), and ovary (9-12%), as well as less frequently involving the small intestines, bile ducts in the liver, brain, and urinary system (all <4%). It appears that there are different cancer rates and different types of cancers related to which of the “mismatch repair genes” have the mutation in Lynch syndrome.
There are two well-accepted screening tests for HNPCC when someone has a cancer that is potentially related to the condition. They both are performed through pathology, and both can help determine whether further genetic testing might be worthwhile. Because of the equal success of the available tests for Lynch syndrome, there has not been consensus as to the best testing approach, so testing is based on institutional preference and resource availability. Because the syndrome is so common, some have recommended testing all patients with colorectal cancer for HNPCC. See below for further discussion of genetic testing for HNPCC.
In terms of colorectal cancer screening and treatment for those patients with HNPCC, colonoscopy screening should start at age 20-25 years (or 10 years before the earliest colorectal cancer in the family) and should be performed every 1-2 years initially and then yearly after age 40. This has been shown to improve survival in patients with the syndrome. If a colon cancer is diagnosed, because of the high risk of developing a second colorectal cancer, there should be consideration of a more extended removal of the colon rather than just the area with the cancer. The surgeon can help weigh the risks and benefits of this. One of the biggest concerns from most patients is how a more extended removal of the colon might impact their bowel frequency, but this has been shown to be well-tolerated by most patients.
Regardless of the amount of colon removed to treat a colon cancer in the setting of HNPCC, the remaining colon and rectum need to be checked yearly with colonoscopy. If a rectal cancer develops, it may be recommended that all of the colon and rectum be removed versus just the part of the rectum involved with the cancer. These options can all be discussed with the surgeon. Screening and treatment for the other cancers associated with HNPCC will not be addressed in this review, but patients must consult with their physician to make sure appropriate tests are done.
Consideration should also be given to a preventative removal of the colon in HNPCC. This is usually based on the very high risks of colorectal cancer noted in a particular family with the syndrome. It may also be considered in circumstances of excessive cancer fears in the patient or difficulties with being able to do appropriate colonoscopies or other tests to try to prevent cancer from developing (or catching it early if it does develop). The surgeon can discuss the risks and benefits of doing this and whether it would be advised. Promising results of studies with the use of aspirin in patients with Lynch syndrome/HNPCC may impact the need for these preventative operations or more extended operations as well.
GENETIC TESTING FOR HEREDITARY COLORECTAL CANCER SYNDROMES
The American Society of Clinical Oncology’s “Guidelines for Genetic Testing” recommend considering genetic testing for a hereditary cancer syndrome when a patient has a personal or family history suggestive of a high risk of having a hereditary cancer syndrome. The guidelines suggest genetic testing be considered when it can be understood by the one who is ordering the test, and the test results will aid in the patient’s diagnosis or help with the management of the patient and/or their family. In addition, someone should be able to offer follow-up care based on the genetic test results. If these criteria cannot be met to a reasonable degree, genetic testing should not be performed.
Genetic counseling is defined as the “…process of helping people understand and adapt to the medical, psychological and familial implications of genetic conditions to disease” (National Society of Genetic Counselors' Definition Task Force). This should be offered whenever genetic testing is being considered (i.e., genetic counseling should always be done before ordering genetic testing). This is especially true when considering genetic testing for hereditary colorectal cancer syndromes. These test results can sometimes be harder to understand than other genetic syndromes, because of the number of potential syndromes, the overlap in symptoms of the various syndromes, the number of genes that may need to be tested, and the frequent need to interpret other tests in addition to gene testing.
The genetic counseling process should include many of the following: discussion of the purpose of the genetic test and information about the genes and who in the family to test; any alternatives to genetic testing; possible test results and how accurate the tests are; the likelihood of an abnormal result; any risks of discrimination based on the results (for example, it could affect someone’s ability to get insurance); psychological issues related to testing and how it may affect someone’s family; confidentiality issues; use of the gene test results to determine any future medical tests or procedures and, if available, preventative measures; and details of the storage and potential reuse of any genetic materials. Interested readers can reference the Aronson article listed at the end of this piece for further details on the subject of genetic counseling.
The companies and institutions that provide gene testing can be found at www.genetests.org, which also provides reviews of the genes and syndromes themselves. Different organizations may perform the tests differently; may require different samples to test (like saliva or blood); may report the results differently; may offer access to genetic counselors to assist in understanding any results; and may contact patients or their care providers when additional tests become available for patients that might have benefited from the tests in the past. For example, they may make contact when a new gene has been identified as being linked to the patient’s syndrome. It is best to discuss with your provider what the options are in terms of test results and how to understand the results once they come back.
The procedure for identifying those to test for a hereditary colorectal cancer syndrome should include the above-described clinical criteria and family histories. If patients have multiple adenomas in the colon, gene testing should focus on APC and MutYH testing, depending on the inheritance patterns in the family and other clinical signs and symptoms (with consideration of MMR gene testing in some circumstances). Hamartomatous polyps should lead to testing for genes as described above: STK11/LKB1, SMAD4, BMPR1A, and/or PTEN. A mixed polyposis assessment could include any of the genes mentioned above in a case-by-case basis.
Because at least 25% of individuals with HNPCC are not going to meet clinical criteria, ideally all colorectal cancers would undergo screening as described above. While clinical and pathology criteria of a tumor can be effective for determining who to test for HNPCC, other prediction models also exist. Details of the gene testing strategies for HNPCC are available in the references.
QUESTIONS FOR YOUR COLON AND RECTAL SURGEON:
- Am I at risk for a hereditary colorectal cancer syndrome?
- Was my cancer or polyps related to a hereditary colorectal cancer syndrome?
- Are the cancers in my family related to a hereditary colorectal cancer syndrome?
- Are there any other cancers or risks associated with my having a hereditary colorectal cancer syndrome?
- When should I have my first colonoscopy and how often should I have them done?
- Are there any other screening tests that I need and how often should I have them done?
- When should my family start colorectal cancer and any other screening?
- Should I or my family have genetic counseling or be considered for genetic testing?
WHAT IS A COLON AND RECTAL SURGEON?
Colon and rectal surgeons are experts in the surgical and non-surgical treatment of diseases of the colon, rectum and anus. They have completed advanced surgical training in the treatment of these diseases as well as full general surgical training. They are well-versed in the treatment of both benign and malignant diseases of the colon, rectum and anus and are able to perform routine screening examinations and surgically treat conditions, if indicated to do so.
DISCLAIMER
The American Society of Colon and Rectal Surgeons is dedicated to ensuring high-quality patient care by advancing the science, prevention and management of disorders and diseases of the colon, rectum and anus. These brochures are inclusive but not prescriptive. Their purpose is to provide information on diseases and processes, rather than dictate a specific form of treatment. They are intended for the use of all practitioners, health care workers and patients who desire information about the management of the conditions addressed. It should be recognized that these brochures should not be deemed inclusive of all proper methods of care or exclusive of methods of care reasonably directed to obtain the same results. The ultimate judgment regarding the propriety of any specific procedure must be made by the physician in light of all the circumstances presented by the individual patient.
CITATIONS AND SELECTED READINGS
Evaluation of Genomic Applications in Practice and Prevention (EGAPP) Working Group. Recommendations from the EGAPP Working Group: Genetic Testing Strategies in Newly Diagnosed Individuals with Colorectal Cancer Aimed at Reducing Morbidity and Mortality from Lynch Syndrome in Relatives. Genet Med. 2009 Jan;11(1):35-41.
Aronson, M. Genetic Counseling for Hereditary Colorectal Cancer: Ethical, Legal, and Psychosocial Issues. Surg Oncol Clin N Am. 2009 Oct;18(4):669-85.
Weissman, S. M., Burt, R., Church, J., et al. Identification of Individuals at Risk for Lynch Syndrome Using Targeted Evaluations and Genetic Testing: National Society of Genetic Counselors and the Collaborative Group of the Americas on Inherited Colorectal Cancer Joint Practice Guideline. J Genet Couns 2012;21(4):484-93.
National Society of Genetic Counselors' Definition Task Force, Resta, R., Biesecker, B. B., et al. A New Definition of Genetic Counseling: National Society of Genetic Counselors' Task Force Report. J Genet Couns. 2006 Apr;15(2):77-83.
Practice Parameters for the Treatment of Patients with Dominantly Inherited Colorectal Cancer (Familial Adenomatous Polyposis and Hereditary Nonpolyposis Colorectal Cancer) Dis Colon Rectum 2003;46(8):1001-1012.
Church, J. “Hereditary Colorectal Cancer.” Chapter in The ASCRS Textbook of Colon and Rectal Surgery, Second Edition. Eds. Beck, D.E., Roberts, P.L., Saclarides, T.J., et al. Springer, 2011:643-68.
Table 1. Amsterdam II Criteria for HNPCC
- ≥3 relatives with an associated cancer (colorectal cancer, or cancer of the endometrium, small intestine, ureter or renal pelvis), one should be a first-degree relative of the other two
- ≥2 successive generations affected
- ≥1 relative diagnosed before age 50 years
- FAP has been ruled out
Table 2: Revised Bethesda Criteria for Testing Colorectal Cancer for Lynch Syndrome
- Patients who meet Amsterdam criteria (above, Table 1).
- Colorectal cancer diagnosed in a patient below age 50 years.
- Presence of synchronous and/or metachronous colorectal or other HNPCC-associated tumors (endometrial, stomach, small bowel, ovarian, pancreas, ureter and renal pelvis, biliary tract, and brain, usually glioblastoma, tumors, sebaceous gland adenomas and keratoacanthomas, and carcinoma of the small bowel), regardless of patient age.
- Colorectal cancer with “MSI histology” (tumor infiltrating lymphocytes, Crohn’s-like lymphocytic reaction, mucinous/signet-ring differentiation, or medullary growth pattern) diagnosed in a patient who is less than 60 years of age.